UF Health patient first to receive new gene therapy for neuromuscular disease since FDA approval
Attention: https://bit.ly/2WwphDi to see a news video about Londyn Wright’s treatment. Visit https://bit.ly/2qFfvUk for a video about how gene therapy works.
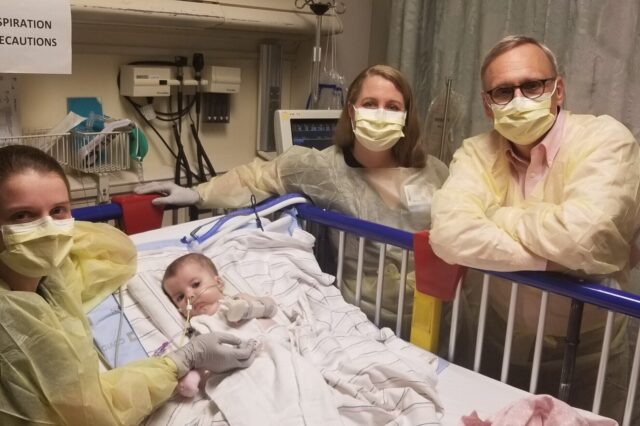
Karen Wright worked her way through the small gathering outside her 4-month-old daughter’s room in pediatric intensive care. It seemed to her as if everyone at UF Health Shands Children’s Hospital had come to witness this historic moment.
Wright, nervous but thrilled, told a nurse, “I think I’m going to cry.”
Near her, a pharmacist delicately held a clear bag containing a plastic syringe. Syringes deliver drugs. But to Wright, this one would do so much more. It would give her daughter, Londyn, the hope of a full, happy life. The pharmacist handed the bag to a nurse who took it into the room, where cartoons danced on a TV screen.
Wright leaned down and whispered to her baby, “This drug is going to save your life.”
On June 7, Londyn became the first baby in the country treated with a newly approved gene therapy for a rare and debilitating neuromuscular disease since it won federal approval in May. University of Florida Health doctors intravenously infused the girl with Zolgensma during an hourlong procedure to treat her spinal muscular atrophy, a rare and deadly disorder that affects the ability to walk, eat and breathe. Untreated, most babies die before reaching their first birthday.
Two researchers associated with UF Health, the university’s academic health center — Nicholas Muzyczka, Ph.D., and Kenneth Berns, M.D., Ph.D. — performed groundbreaking work in the 1980s on adeno-associated virus, or AAV, that made Londyn’s treatment possible, said Barry J. Byrne, M.D., Ph.D., who led Londyn’s treatment team. It is the second time in recent years that techniques pioneered at the University of Florida have led to new gene therapies. In December 2017, a therapy developed in part by ophthalmology researcher William Hauswirth, Ph.D., won federal regulators’ approval to treat a genetic form of vision loss known as Leber congenital amaurosis type 2.
“This is very exciting,” said Byrne, director of the UF Powell Gene Therapy Center and a pediatrics professor in the UF College of Medicine. “As a pediatrician, I’m particularly grateful to see things we’ve been working on in the lab for many years impacting pediatric care. And pediatricians of the future will be able to use this therapy without having to deliver such bad news to the families of these wonderful children.”
Spinal muscular atrophy, or SMA, is the leading genetic cause of infant death. It affects about 1 in 11,000 babies, according to Cure SMA. The U.S. Food and Drug Administration approved Zolgensma on May 24. It works by using a small, harmless virus to deliver functional copies of a gene that is mutated or missing in SMA patients. A dysfunctional gene, known as survival motor neuron 1, causes nerve cells to malfunction and die. That leads to chronic and often fatal muscle weakness.
“When I found out it was SMA, I thought I was going to lose my baby,” said Wright. “Now, who knows what the future holds. That is still unpredictable. But I think she’s going to be a normal little baby, running around, fighting with her brothers. She’s going to be fine.”
UF Health’s role in delivering the treatment exemplifies its commitment to world-class patient care and pioneering work on gene therapies, said David R. Nelson, M.D., senior vice president for health affairs at UF and president of UF Health.
“Our devotion to patient care and expertise in gene therapy and other scientific research is an exceptional combination of capabilities. It is extremely gratifying that patients who need complex treatments put their trust in UF Health,” Nelson said.
A problem emerges
Karen Wright and husband David first noticed a problem with her daughter about two months after her birth. The bright-eyed, observant little girl became lethargic and weak. Her legs didn’t move like most babies and her development seemed slowed. Then, she developed aspiration pneumonia, which doctors later said was a consequence of SMA. Wright, who lives in Blountstown west of Tallahassee, took Londyn to a Panama City hospital.
Londyn would be hospitalized two times before her pediatrician, suspecting SMA, ordered a test and confirmed the diagnosis about May 21. The pediatrician then referred the baby to UF Health.
What followed was a lesson in fortuitous timing. On May 24, Londyn had an appointment at the UF Health Center of Pediatric Neuromuscular and Rare Diseases with Carla Zingariello, D.O. The physician talked to Wright about a remarkable new drug, Zolgensma, that might offer the hope of a normal life for Londyn.
Zingariello told the mother that FDA approval was expected by the end of May. But who knew when, really? Would it be two weeks or two months? “I couldn’t tell her when that would happen,” Zingariello said.
Meanwhile, Londyn’s health would be deteriorating. The only other option would be Spinraza, a drug which required four injections the first two months via a spinal tap and injections every four months for the rest of the patient’s life. Zolgensma, on the other hand, would be a one-time infusion.
Wright went to lunch after the appointment. Her cell phone rang. It was Zingariello.
“You’re not going to believe this,” the doctor told her. “I just got a call and the FDA approved the gene therapy today, like 10 minutes ago.”
Wright immediately returned with Londyn to the clinic for a test required to ensure her body would not fight back against the gene therapy. She passed.
Kara Godwin Wild, a pediatric nurse practitioner and care coordinator in the UF Health Center of Pediatric Neuromuscular and Rare Diseases, contacted the family’s insurer the same day the drug was approved. Londyn had coverage through a Medicaid program.
Time was critical. Every day that Londyn’s condition progressed meant the loss of motor neurons that could not be replaced.
“I called right away because I knew the process for approval could be a long and hard road,” Godwin Wild said. “And every single day, Londyn loses motor neurons. She was getting weaker and weaker. This was a race.”
Infusion of hope
On June 6, the night before Londyn’s infusion, a cooler containing four vials of Zolgensma was flown from Chicago to Orlando by a drug company representative. Another rep drove it from Orlando to Gainesville.
This was no ordinary cooler. For one, it was lined with dry ice and outfitted with wireless tracking that included temperature controls monitored in Illinois. Zolgensma is produced by AveXis, which is owned by the Swiss pharmaceutical company Novartis.
The cooler arrived safely and was transferred to Carrie Lagasse, Pharm.D., a clinical pharmacy specialist for UF Health Shands. At 6 p.m. Lagasse texted Godwin Wild, “Signed. Sealed. And delivered.”
The drug company representative who drove the Zolgensma from the airport tracked down Godwin Wild at a local restaurant where the UF Health employee was enjoying a meal with her family.
The pair hugged.
Before the infusion, UF Health pharmacy staff thawed the vials overnight. In a sterile room the next day, a steady-handed Lagasse drew the contents out of the vials and into a syringe pump.
“I was pulling out vials … and realized that any error would be unacceptable,” said Lagasse, who compounded the drug. “So, I was a little shaky at first.”
But in 10 minutes, the job was done. The drug had been transferred to a large plastic syringe.
Later in the day, Londyn received the gene therapy. As the Zolgensma flowed into her body, her mother, wearing a protective gown and mask, offering her baby reassuring words.
“I was just telling her that this is going to help you,” Wright said later. “You’re going to have a normal life. You’re not going to be hooked up to these machines forever. This is all temporary and you’re going to have a good life. This drug is going to save you. And I love you so very much.”
Caring for Londyn was a true team effort: The Pediatric Intensive Care Unit staff kept watch over her day in and day out, making sure she was stable enough to receive the gene therapy. The team included Lara Nicolas, M.D., Londyn’s critical care physician and Krystyn Linville, a pediatric critical care nurse practitioner.
Doctors have told Karen Wright that it may be several weeks before she notices any difference in her child. And Byrne said that since the drug is so new, the long-term ability of Londyn’s body to fight SMA is still uncharted territory. The hope, he said, is that she will lead a normal life.
"But I think the indications are that many of the deficits she has now will recover,” Byrne said.
UF Health Shands CEO Ed Jimenez said being able to offer families the hope of a better, healthy life is a guiding principle for the hospital’s caregivers.
“Seeing this beautiful baby reminds us all why we work in medicine,” he said. “It’s so very important that we offer our very best to ensure Londyn has a fighting chance at a healthy and happy future.”
Wright said she is thankful for the excellent care Londyn’s care team has provided and still trying to wrap her brain around how everything from FDA to insurance approval just fell rapidly into place.
“Everything that’s happened,” said Wright, “it’s just beyond me.”
UF Health science writer Bill Levesque contributed to this story.
About the authors

