CO2 blood test
Definition
CO2 is carbon dioxide. This article discusses the laboratory test to measures the amount of carbon dioxide in the liquid part of your blood, called the serum.
In the body, most of the CO2 is in the form of a substance called bicarbonate (HCO3-). Therefore, the CO2 blood test is really a measure of your blood bicarbonate level.
Alternative Names
Bicarbonate test; HCO3-; Carbon dioxide test; TCO2; Total CO2; CO2 test - serum; Acidosis - CO2; Alkalosis - CO2
How the Test is Performed
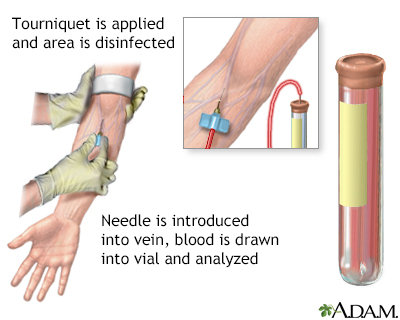
A blood sample is needed. Most of the time, blood is drawn from a vein located on the inside of the elbow or the back of the hand.
How to Prepare for the Test
Many medicines can interfere with blood test results.
- Your health care provider will tell you if you need to stop taking any medicines before you have this test.
- DO NOT stop or change your medicines without talking to your provider first.
How the Test will Feel
You may feel slight pain or a sting when the needle is inserted. You may also feel some throbbing at the site after the blood is drawn.
Why the Test is Performed
The CO2 test is most often done as part of an electrolyte or basic metabolic panel. Changes in your CO2 level may suggest that you are losing or retaining fluid. This may cause an imbalance in your body's electrolytes.
CO2 levels in the blood are affected by kidney and lung function. The kidneys help maintain the normal bicarbonate levels.
Normal Results
The normal range is 23 to 29 milliequivalents per liter (mEq/L) or 23 to 29 millimoles per liter (mmol/L).
Normal value ranges may vary slightly among different laboratories. Talk to your provider about the meaning of your specific test results.
The example above shows the common measurement range of results for these tests. Some laboratories use different measurements or may test different specimens.
What Abnormal Results Mean
Abnormal levels may be due to the following problems:
Lower-than-normal levels:
- Addison disease
- Carbonic anhydrase inhibitors (used to treat glaucoma)
- Diarrhea
- Ethylene glycol poisoning
- Ketoacidosis
- Kidney disease
- Lactic acidosis
- Metabolic acidosis
- Methanol poisoning
- Renal tubular acidosis -- distal
- Renal tubular acidosis -- proximal
- Respiratory alkalosis (compensated)
- Salicylate toxicity (such as aspirin overdose)
- Ureteral diversion
Higher-than-normal levels:
- Bartter syndrome
- Burns
- Cushing syndrome
- Excessive sweating
- Hyperaldosteronism
- Metabolic alkalosis
- Respiratory acidosis (compensated)
- Syndrome of inappropriate diuretic hormone secretion (SIADH)
- Vomiting
Delirium may also alter bicarbonate levels.
Gallery
References
Ring T. Acid-base physiology and diagnosis of disorders. In: Ronco C, Bellomo R, Kellum JA, Ricci Z, eds. Critical Care Nephrology. 3rd ed. Philadelphia, PA: Elsevier; 2019:chap 65.
Seifter JL. Acid-base disorders. In: Goldman L, Schafer AI, eds. Goldman-Cecil Medicine. 26th ed. Philadelphia, PA: Elsevier; 2020:chap 110.